You’ve probably seen videos of spacecraft like the Space Shuttle or the SpaceX capsule come back to Earth. Whether you see it from the ground or from inside the spaceship one thing is abundantly clear. It’s hot. And I know that might be counterintuitive because of how cold space is (-455 degrees Fahrenheit) but the second that ship hits the atmosphere it goes up in flames. This, at its simplest, is because of air resistance. You know, that thing that slows you down while riding your bike and makes your car way less fuel-efficient than it could be. Air resistance in our daily lives is frustrating, to be frank, but when getting back from space, it’s a life-saver.
Air resistance happens because air is something. It’s easy to think it’s not there because we can’t really see or feel it like we do everything else. But every whisp of wind that hits your face is literally a gas running into you, or you running into it. Think of being in a pool. When you try to move your arm underwater, it’s way harder than if you were to lift it above the surface. But even then, it’s harder than it could be. In space, there’s nothing to slow you down and, while it wouldn’t be as big of a difference as in water, it would definitely be noticeable. In a few other newsletters, we discussed the idea of drag, and how to calculate it. Most simply it’s the equation below.
If you’re confused, don’t be. Simple as that. Or just look at this picture. A is an object’s surface area, V is its velocity, rho (the ‘p’ looking thing) is the air’s density, and C_D is the coefficient of drag—this is found through testing and is usually a number between 0.5 and 5. The lower this number is, the more aerodynamic the object is.
So, putting the equation and figure together, the larger something is (more surface area), or the faster the air is hitting it, the more drag the object will experience. Or, in our case from earlier, if the medium it’s traveling through is denser (i.e. water is denser than air), the harder it will be to move. This is why your arms move more slowly underwater and also why you can’t swim in air.
In general, this drag force slows the object down. When re-entering from space, going 15,000 mph, that’s exactly what we need to do, and fast. We can’t afford to carry enough fuel to do it ourselves, so we have to use the air. As we slow down, we lose kinetic energy. This is simply energy that an object has due to movement—the faster it goes the more energy it has; however, this energy doesn’t just disappear. Thanks to a lovely law called the Conservation of Energy, energy can’t be created or destroyed. It’s gotta go somewhere. In the case of drag, this energy becomes heat. And, like we touched on, there’s a whole lot of heat to come from slowing down this quickly. We can do a quick back of the hand calculation so we don’t have to get too deep into thermodynamics…
Kinetic energy is simply:
V is the object’s velocity (speed) and m is the object’s mass. The heavier or faster the object is, the more kinetic energy it has. I know what you’re thinking: “why do all of these equations have a ½?” Well, my friend, that’s because of calculus. Remember when you take the integral the exponent has to come down as a fraction? Neither do I, forget it.
Regardless, we can examine the change in energy that the spacecraft experiences by simply taking the difference. Before it starts to slow down minus after.
In our case, the mass of the spacecraft doesn’t change. Substituting in the mass (let’s assume it’s the SpaceX dragon capsule) of ~9,600 kg (21,000 lbs), the speed in orbit of 27,000 km/hr (17,000 mph), and the velocity the capsule needs to slow to in order to open its parachute of 560 km/hr (350 mph), we get the difference in kinetic energy before and after this event is 270 million kJ. [1] This process takes 7 minutes. Therefore, the capsule loses kinetic energy to heat at a rate of 643,000 kJ/second. That’s enough energy to boil 563 gallons of water every second. This is great information, but no one can really visualize how much heat that is. We need to figure out what temperature this translates to. This can be done with a simple equation from Thermodynamics that relates heat load to temperature.
Q is the heat load, which we already found, m is the mass of the air that’s affected, delta T is the change in temperature, and c is a constant (depends on the material being heated—in our case it’s air). At the highest temperature point of flight, the capsule is hitting about 300 kg of air every second. [2] That calculation is a lot more… fun… so we don’t have to worry about it here. This gives us the result that, every second, the capsule is heating the air from almost zero, to 2,150 degrees Kelvin, or 3,410 degrees Fahrenheit. That’s a third of the temperature of the Sun. We hot. Interestingly, the temperature of the capsule during its actual re-entry was reported to be 3,500 degrees Fahrenheit. [1] I’m just as surprised as you are that we got that close—if not more.
Now that this is clear as mud, we have to figure out how to protect against this kind of heat. We’ve proved it’s necessary, so we’ll just plan around it. This is done with what are known as heat shields. These are protective layers on the spacecraft made of extremely heat-resistant materials, often ceramics. They’re actively cooled down with air or oil that gets passed through them. Here’s what they look like on the SpaceX capsule: [3]
As you can see, even though they work, they still get burnt to a crisp… can you blame them? They manage to withstand 3,500 degrees while keeping the inside a chilly 75; just over a foot away. Safe to say they’ll suffice as literal sunblock for our spaceship.
In conclusion, we’ve successfully managed to stumble upon how hot spaceships get as they re-enter the atmosphere. While doing so, we got to learn about conservation laws, energy, and thermodynamics! Why don’t you look excited? Anyway, thank you for reading this week’s newsletter and I’ll see you next week for more. Don’t forget to subscribe and as always please comment with questions or ideas for future newsletters!
Check out last week’s newsletter here.
Special thanks to Ian MacDonald for inspiration.
For more details…
[1] https://www.nasa.gov/feature/top-10-things-to-know-for-nasa-s-spacex-demo-2-return
[2] https://www.spacex.com/vehicles/dragon/
[3] https://www.teslarati.com/spacex-crew-dragon-heat-shield-reentry/
Cover Image: https://www.nasaspaceflight.com/2021/01/dragon-departs-iss-with-science/




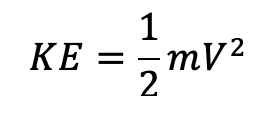



Difficult subject made easier by McDonald's ability to bring it to the average person.
Great article. one question... How does the capsule keep the heat shield facing toward the air rushing at it?